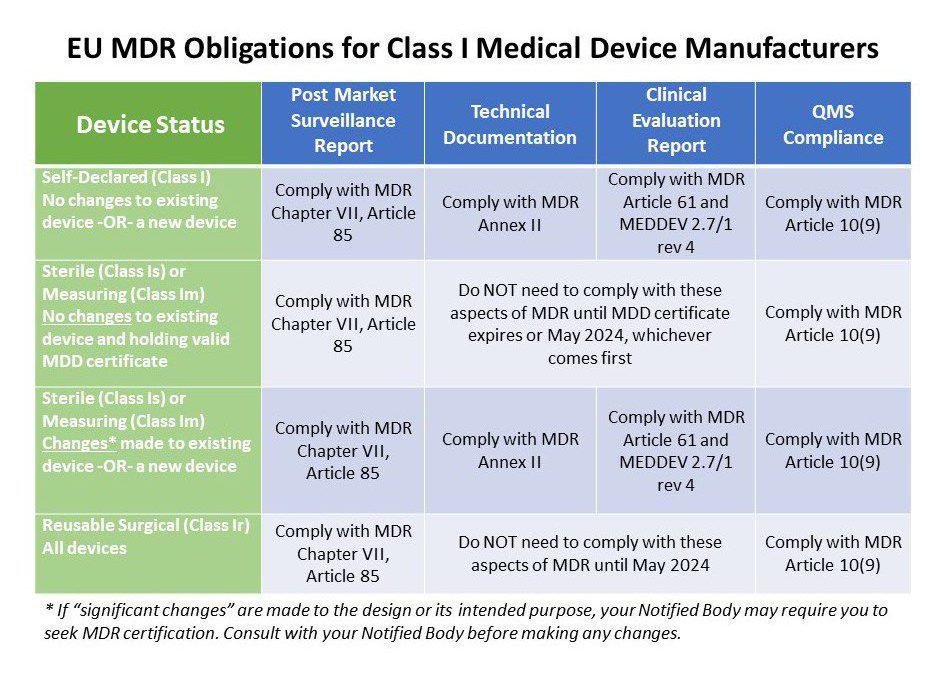
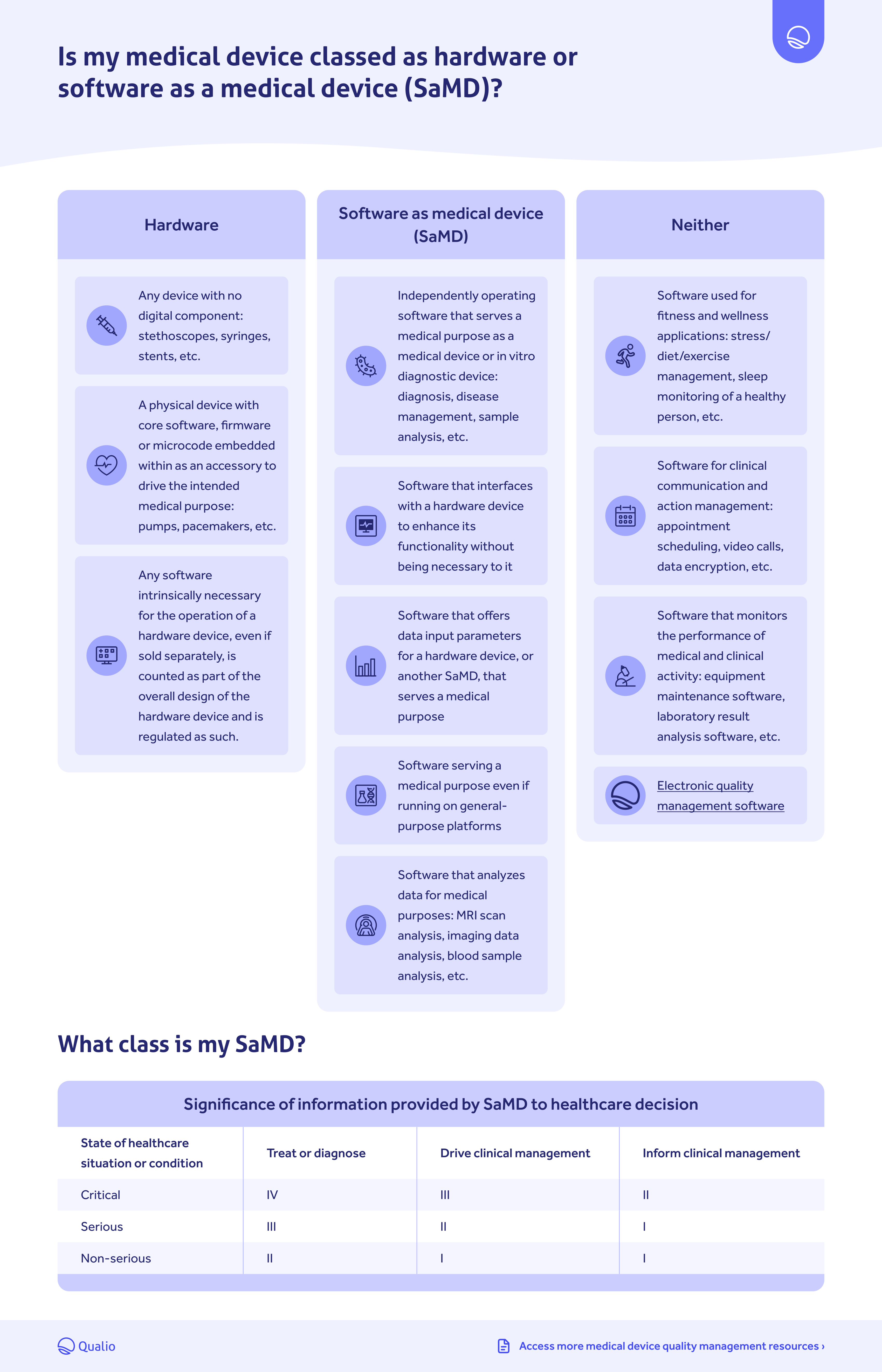
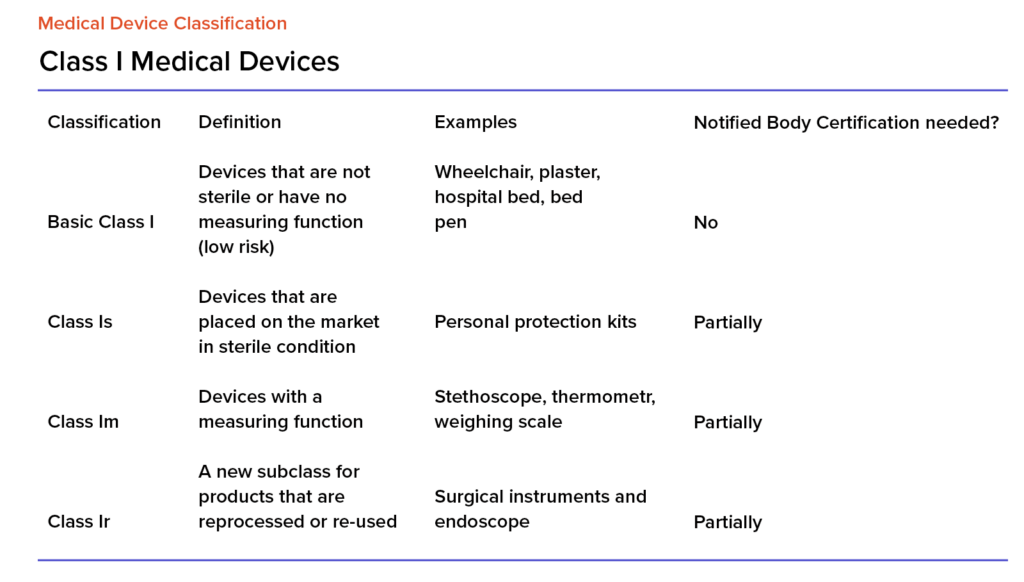
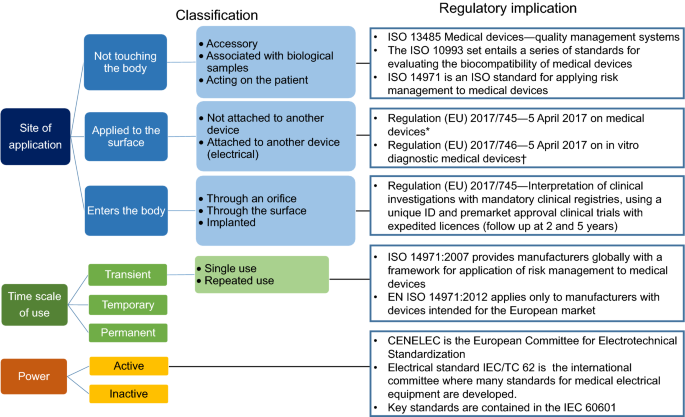
What are the Essential Requirements for Medical Device CE Marking? - Medical Device Academy Medical Device Academy

HOW TO BRING A MEDICAL DEVICE TO MARKET IN EUROPE - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal

CONSIDERATIONS FOR SURGICAL MEDICAL DEVICE TRIALS LCTU Liverpool Clinical Trials Unit Considerations for Medical Device Trials. - ppt download

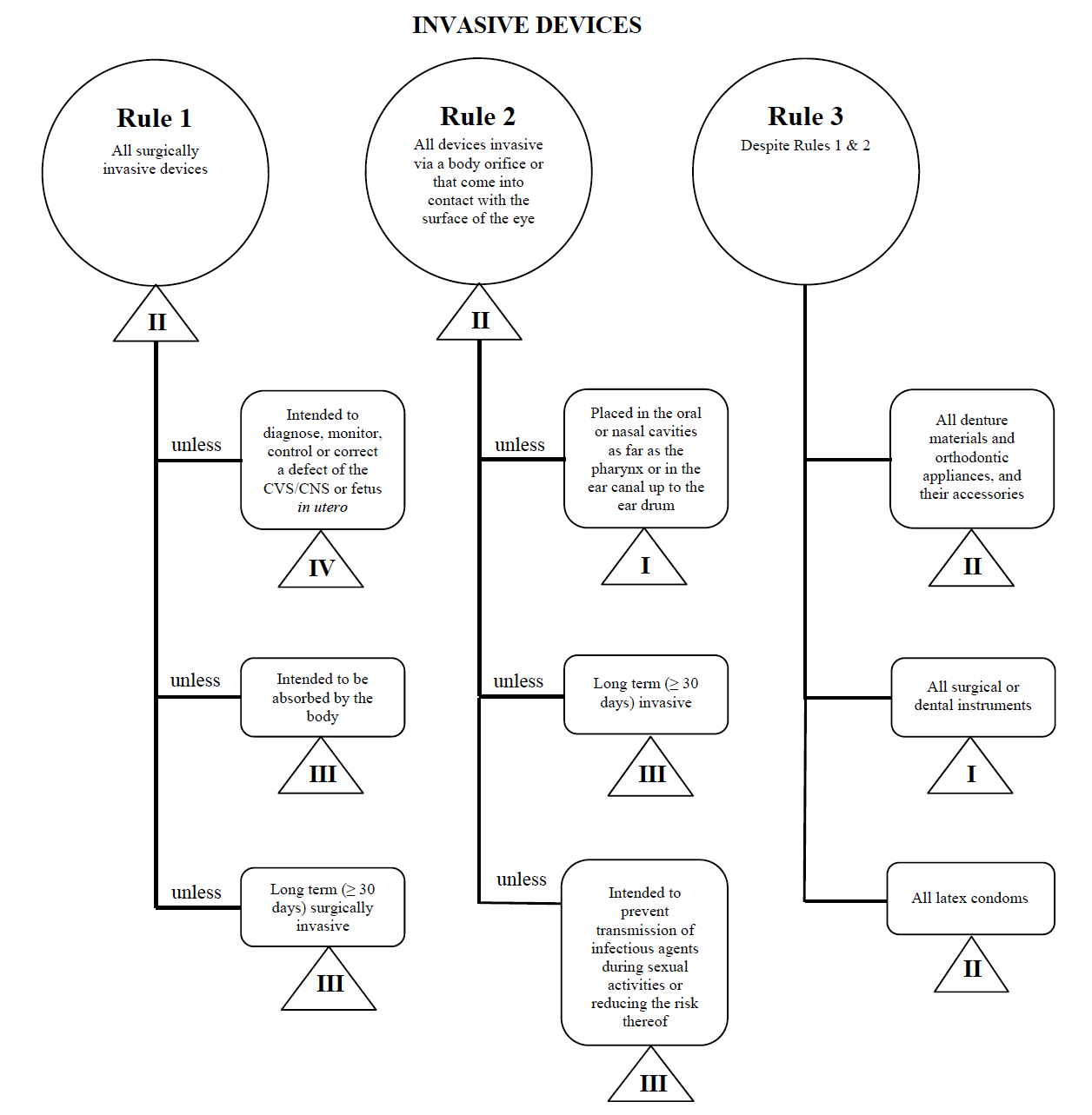

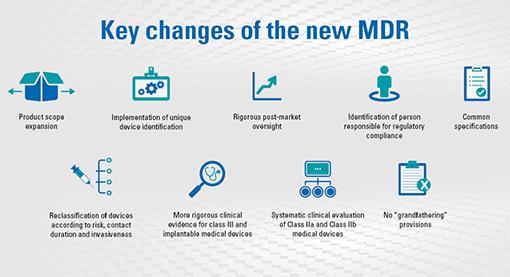


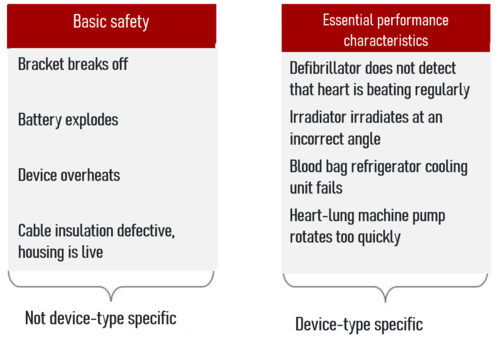




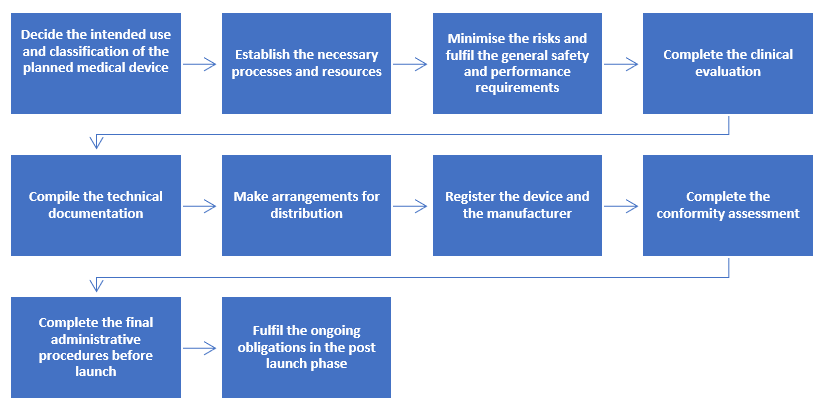
![PDF] Trustworthy Medical Device Software | Semantic Scholar PDF] Trustworthy Medical Device Software | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/11d47fe3ecc93d8ba9526c9426b1480a8ea66461/3-Table1-1.png)