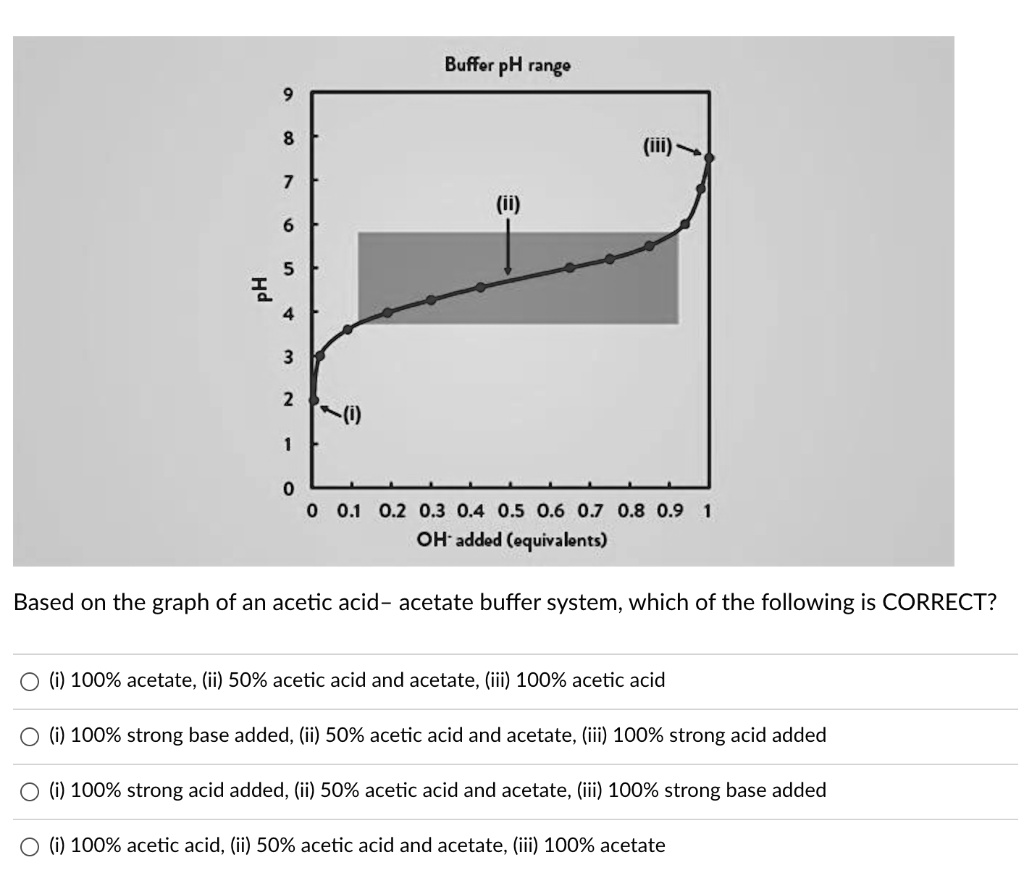
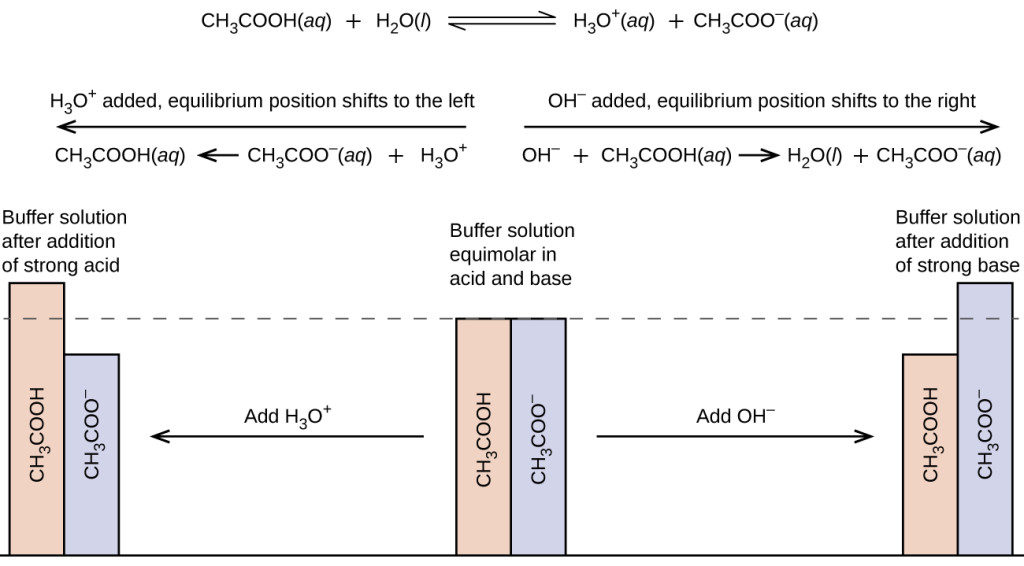
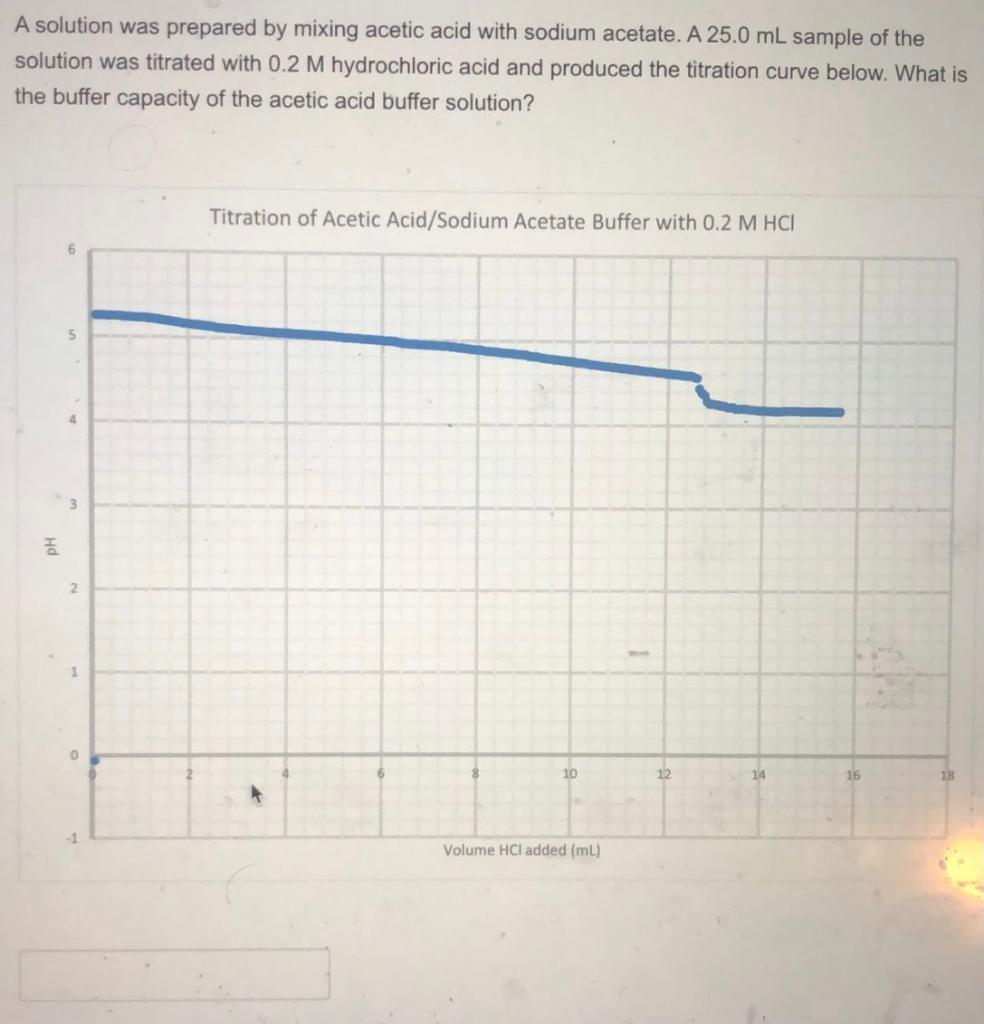
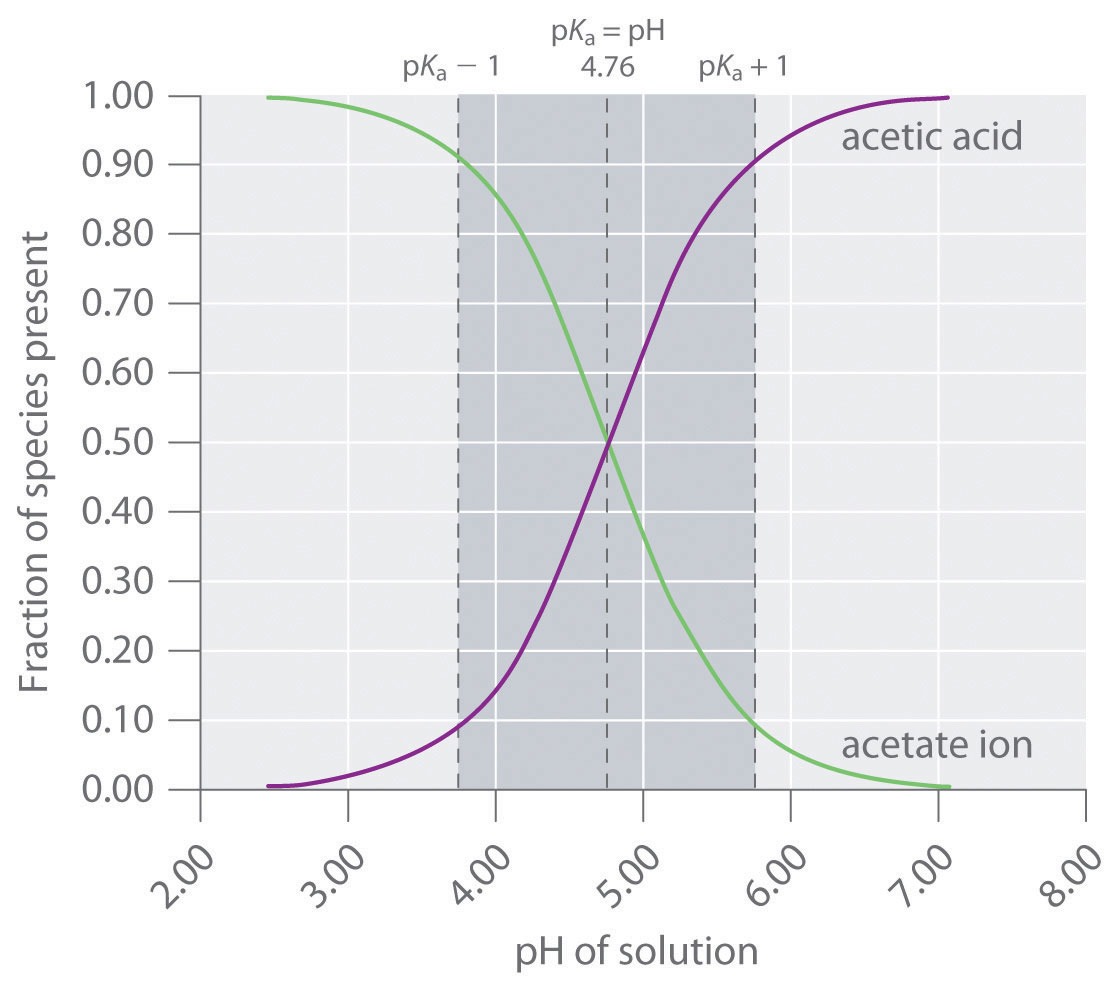
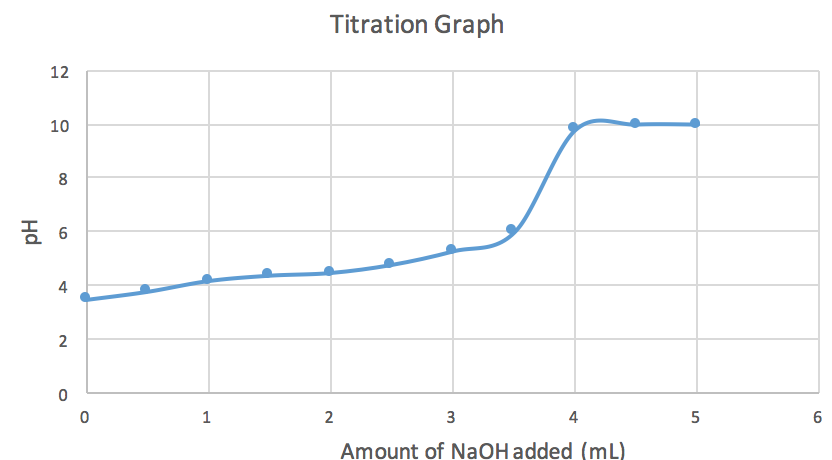
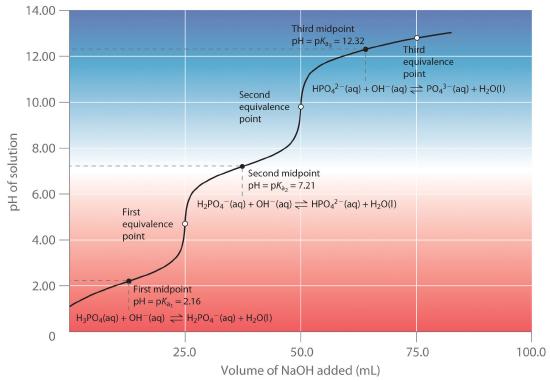
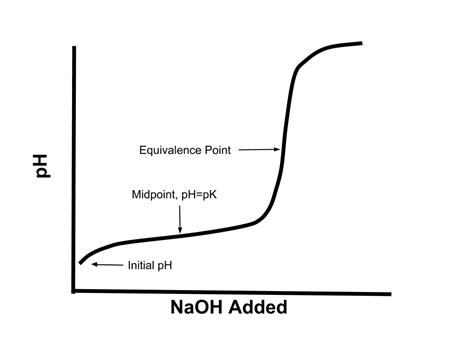
A solution of acetic acid is titrated with NaOH. Explain how the pK_a of acetic acid can be found using a titration curve. | Homework.Study.com
a) V-G curve for equal molar sodium acetate/acetic acid buffer with a... | Download Scientific Diagram

Exp 5 Determination of the Buffer Capacity of Acetic Acid and Sodium Acetate Buffer System - Studocu

SOLVED: How would you prepare 250 mL of a 200 mM acetate buffer at pH 4.0 using sodium acetate trihydrate crystalline (MW: 136.0 g/mol) and solution of 200 mM HCI (pKa for

Titration of CH3COONa with HCl and pKa determination from half equivalence point - Chemistry Stack Exchange
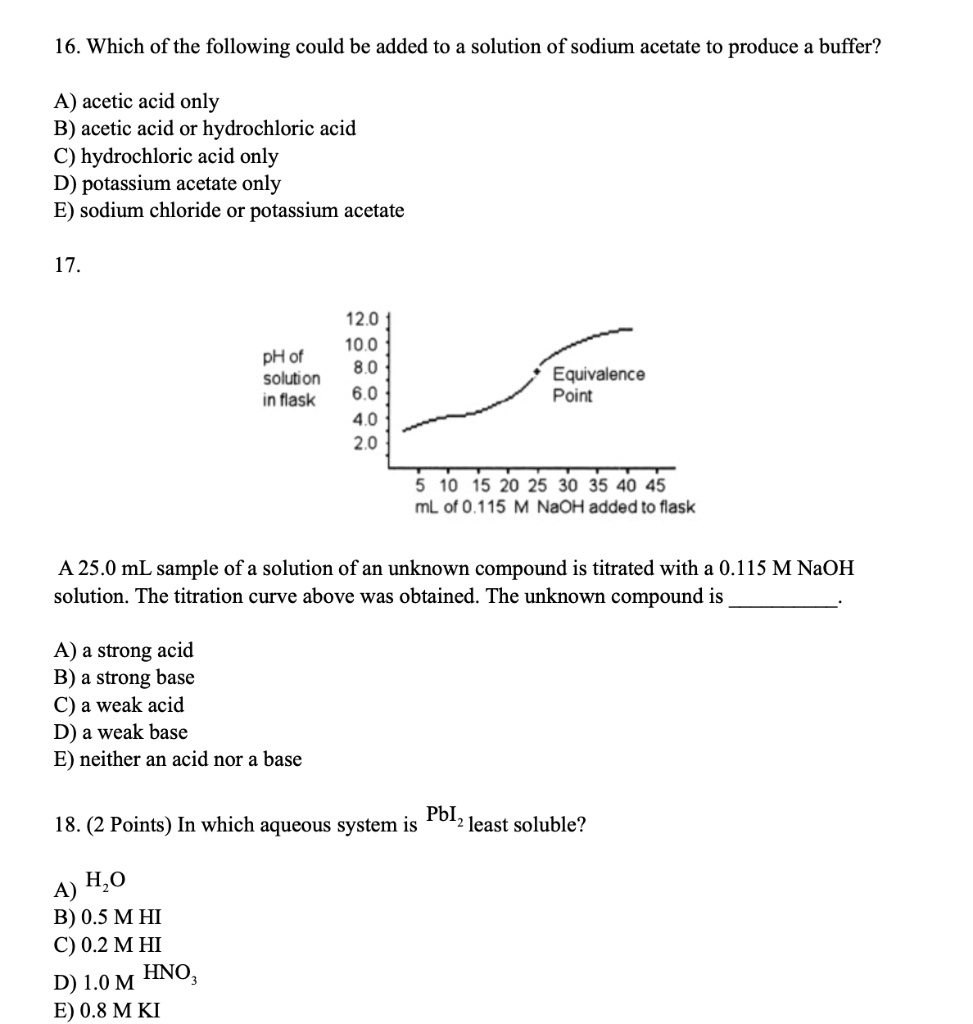
SOLVED: 16. Which of the following could be added to a solution of sodium acetate to produce a buffer? A) acetic acid only B) acetic acid or hydrochloric acid C) hydrochloric acid

Maths and Chemistry for Biologists. Chemistry 4 Buffers This section of the course covers – buffer solutions and how they work the Henderson-Hasselbalch. - ppt download

Consider the titration of sodium hydroxide solution (0.100 M) with acetic acid (0.100 M), i.e. a weak acid-strong base titration. a) Sketch the titration curve for such a reaction. Label the curve.

Solubility of Sodium Acetate in Binary Mixtures of Methanol, 1-Propanol, Acetonitrile, and Water at 298.2 K | Journal of Chemical & Engineering Data
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora